¶ In a note published in the Society of Biology on November 19, 1919, d’Hérelle indicated a method for measuring Bacteriophage. He puts 1/50,000 teaspoon of Bacteriophage into 10 c.c. of an emulsion containing approximately 250 million microbes per teaspoon and spreads a scoop of this mixture (i.e. 1/100 c.c.) onto a slanted agar tube. He then counts the number of dissolution areas and estimates from this the number of ultramicrobes. In fact, the Bacteriophage does not act like a liquid, exerting the same action over the entire seeding surface; lysis only occurs at locations where an ultramicrobe has been deposited on the agar.
¶ This method is, however, rather difficult to carry out because the clarification ranges [plaques?] can be extremely small and therefore not very apparent. Let us add to this that, when they are somewhat large, we could suppose that some of them result from a lack of seeding at this level [I’m unsure what this means].
¶ For the dosage, we tried the process of successive dilutions recommended by Miquel for water analysis. For this purpose we introduce, into tubes seeded with the microbe capable of undergoing lysis, decreasing quantities of Bacteriophage 1/10, 1/100, 1/1000, up to 1/1,000,000,000,000 [that’s 1/1 trillion or 1/1012!], according to that the microbe has normal development or undergoes immediate or subsequent lysis we admit the presence or the absence of the Bacteriophage in question.
¶ When we dose the Bacteriophage coming from tubes, inoculated with the same number of drops of Bacteriophage and the same number of drops of culture, we constant on both sides the same drops of culture [this too is phrasing I struggle with], we note on both sides the same activity. However, it happens from time to time that a given dilution provides a negative seeding for Bacteriophage, while the dilution ten times stronger still contains some. This is explained quite well with the notion of Bacteriophage presuming a living being. We also observe similar facts in the counting of water germs using Miquel’s method.
¶ This technique allowed us to make the following observations:
¶ 1. The Bacteriophage introduced into broth inoculated with lysable microbes, increases quantitatively during the stay in the oven [I assume this is an oven incubator]. This increase is approximately the same for a Bacteriophage and a given culture, whether or not the seeding was massive with the lysable culture or with the Bacteriophage, whether there was immediate inhibition on development or late lysis.
¶ 2. The centrifugation pellet of the Bacteriophage (8,000 revolutions per minute for one hour) does not contain more active elements than the upper liquid.
¶ 3. Certain chemical influences, which apparently leave the Bacteriophage intact, nevertheless considerably reduce its activity when the effect produced by these agents is examined by the dosage method. So when we put the Bacteriophage in contact with alcohol at 50 p. 100 or with 5 p. carbolic acid. 100 reviews massively we find [I don’t understand this phrasing]. In these filtrates, Bacteriophage is still active, but with the dosage method we see that the content is considerably reduced, as can be seen from the attached table:
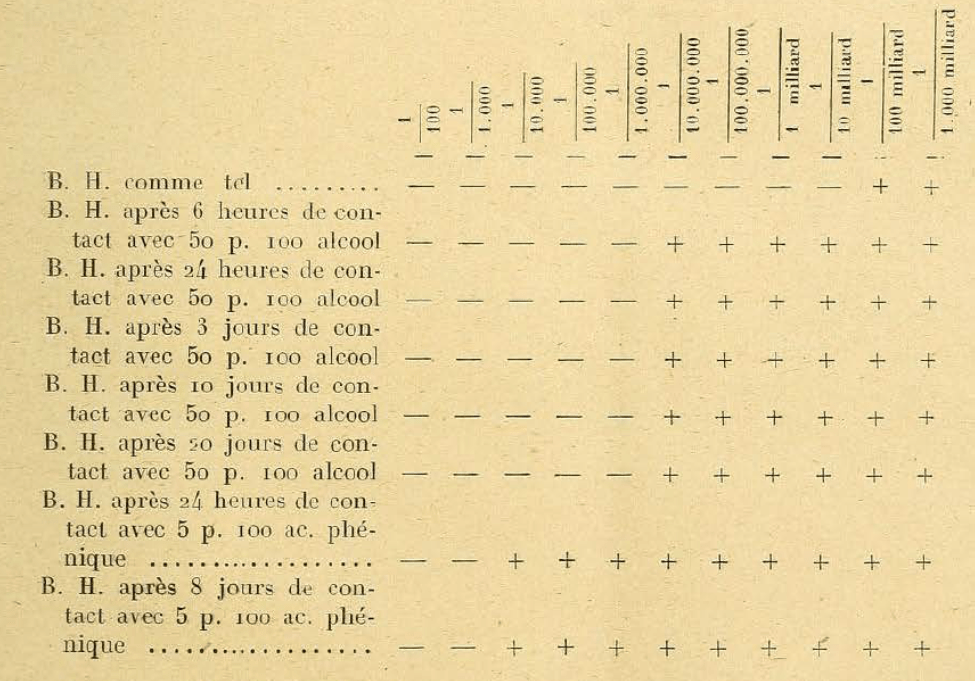
¶ Note. — B. H. means Bacteriophage d’Herelle; — means absence of development of the microbe and presence of Bacteriophage; + means normal development of microbes and absence of Bacteriophage. Fact worthy of being noted: under the influence of alcohol and carbolic acid, the reduction of Bacteriophage occurs quickly, without being progressive: it stops at a certain level, as if the filtrate contained a certain number of elements more resistant to the harmful agents in question.
¶ (Bacteriology Laboratory of the University of Louvain).

